Atomic Radius Across a Period
Can be borrowed from the vacuum for a period of time. They allow energy densities up to 10 7 Wcm 2 across a.
The McClelland royal commission showed that the British were cavalier about the weather conditions during the bomb tests and that fallout was carried much further than the 100-mile radius agreed.
. It is estimated that between 40000 and 75000 people died immediately following the atomic explosion while another 60000 people suffered severe injuries. Ionic radius decreases moving from left to right across a row or period. There are many trends on the periodic table.
The definition of electronegativity is. Across a period the number of electron shells remains the same while the number of electrons increases. A plane section of a right circular cone that.
The possible decay particles in the sequence are. Within a period of elements each new electron is added to the same shell. A closed plane curve generated by a point moving in such a way that the sums of its distances from two fixed points is a constant.
The tendency of an atom to attract electrons to form a chemical bond. The radius of total destruction from the atomic blast was about one mile followed by fires across the northern portion of the city to two miles south of where the bomb had been dropped. Atomic radii increase toward the bottom left corner of the periodic table with Francium having the largest atomic radius.
1 2. Atomic Radius Trend 1. Between 90000 and 166000 people are believed to have died from the bomb in the four-month period following the explosion.
Lets break down the trend into its period and group trends. Trends in Ionic Radius Across a Period. At the atomic-level.
This is because Chlorine has a larger. Atomic radius decreases across the period. And all the XAFS data were collected during one period of beam time.
When an electron is added a new proton is also added to the nucleus which. The electronegativity of an atom depends upon its atomic number and its atomic radius which means that the more the distance between the nucleus and its valence electrons the lower the electronegativity and vice versaElectronegativity in the period table. Thanks for the list quite helpful.
More protons are added but the outer valence shell remains the same so the positively charged nucleus draws in the electrons more tightly. This is because the effective positive force of the nucleus also increases drawing in the electrons more tightly. This is because the starting elements in a period tend to form cations and the elements.
The first atomic radius periodic trend is that atomic size decreases as you move left to right across a period. Were homogeneously distributed across the entire N-C framework in Ru-N-C 3334. Even though the size of the atomic nucleus increases with larger atomic numbers moving across a period the ionic and atomic radius decreases.
Atoms decrease in size across the period and increase in size down the group. Thus the increasing number of nucleus attracts the more electrons more tightly towards it and the atomic radius decreases. The issue of the radius of the electron is a challenging problem of modern theoretical physics.
In one time period become maximum minimum two time So It means frequency of is 2. Department of Energy has estimated that after five years there were perhaps 200000 or more fatalities as a result of the bombing. Ionic Radius and Group.
Moving from left to right across a period the number of protons and electrons increases while the number of energy shells stay same. While the atomic radius follows a similar trend ions may be larger or smaller than neutral atoms. The first atomic bomb Little Boy was dropped on Japan on August 6 1945.
For example Sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. Let us understand the trends in the ionic radius of elements across a period with an example. Atomic Radii Decrease From Left to Right Across a Period.
4 thoughts on A Z of Science Fiction words Chris Higgins December 15 2018 at 401 am. A radioactive nucleus EX undergoes spontaneous decay in the sequence Z EX Z1B Z3C Z2D where Z is the atomic number of element X. As you move from left to right across an element period row the ionic radius decreases.
Why does the atomic radius decrease across a period. Suggested changes corrections Ecliptic to Elliptic. In period 3 we find that the atomic radius first decreases and then suddenly increases and then again it slowly decreases.
In atomic physics the Bohr model or RutherfordBohr model presented by Niels Bohr and Ernest Rutherford in 1913 is a system consisting of a small dense nucleus surrounded by orbiting electronssimilar to the structure of the Solar System but with attraction provided by electrostatic forces in place of gravityIt came after the solar system Joseph Larmor model 1897 the. The admission of the hypothesis of a finite radius of the electron is incompatible to the premises of the theory of relativity.
What Is The Trend In Atomic Radius As You Go Across A Period Socratic
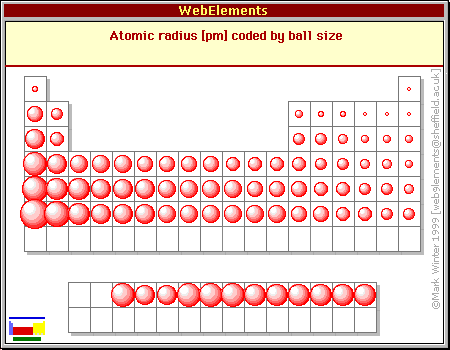
How Does Atomic Radius Change From Left To Right Across A Period In The Periodic Table Socratic
How Does Atomic Radius Change As You Move Across The Periodic Table Quora

Chemistry Periodic Variations 7 Of 23 Atomic Radius What Determines The Radius 3rd Period Youtube
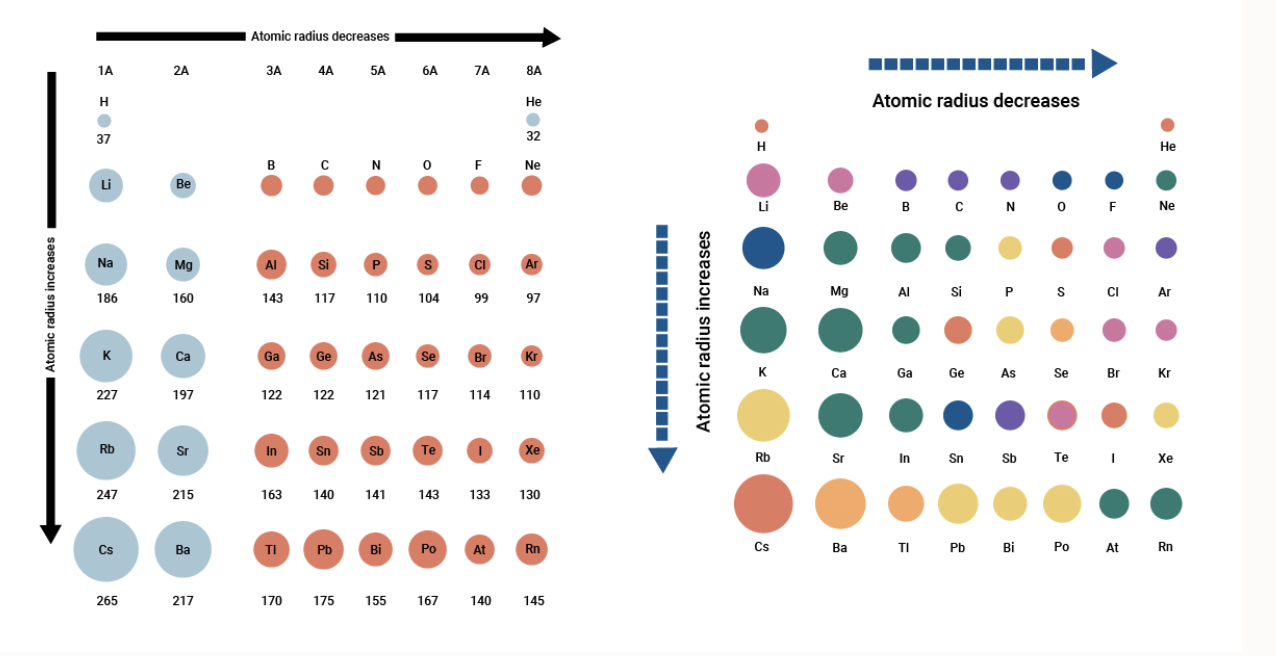
0 Response to "Atomic Radius Across a Period"
Post a Comment